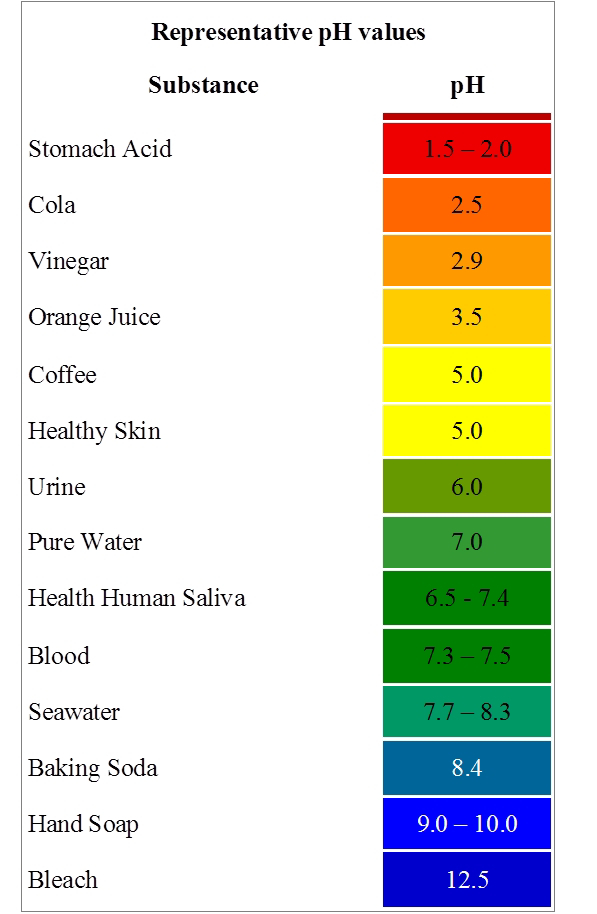
In 1909 S.P.L Sorenson, a Danish biochemist devised a scale known as pH to represents the H+ ion concentration of an aqueous solution. The pH value of any solution is a number that simply represents the acidity and basicity of the solution. The pH value of any solution is numerically equal to the logarithm of the inverse of the hydrogen ion (H+) concentration. Hence, the pH solution is referred to as the negative logarithm of hydrogen ion.
pH of Neutral Solution (Pure Water): pH of water is 7. Whenever the pH of a solution is 7, it will be a neutral solution. Such a solution will have no effect on any litmus solution or any other indicator.
pH of an Acidic Solution: All the acidic solutions have a pH of less than 7. So, whenever a solution has a pH less than 7, it will be acidic in nature and it will turn blue litmus into the red as well as methyl orange pink and phenolphthalein colourless.
pH of a basic solution: All the alkaline solution has a pH of more than 7. So, whenever a solution has more than 7 values then it will be basic in nature and it will turn red litmus to blue, methyl orange to yellow and phenolphthalein to pink.





No comments:
Post a Comment